Nerivio Can Greatly Reduce Payer Expenses Through Reduction of Migraine Headache Days, Productivity Loss, and Healthcare Utilization Costs
NETANYA, Israel and BRIDGEWATER, N.J., May 16, 2023 /PRNewswire/ — Theranica, a prescribed digital therapeutics company developing advanced neuromodulation devices for migraine and other pain conditions, announced the results of a recent study published in the Journal of Medical Economics, showing that Nerivio®, a novel Remote Electrical Neuromodulation (REN) wearable, provides significant cost savings in addition to clinical benefits when used as a preventive migraine treatment.
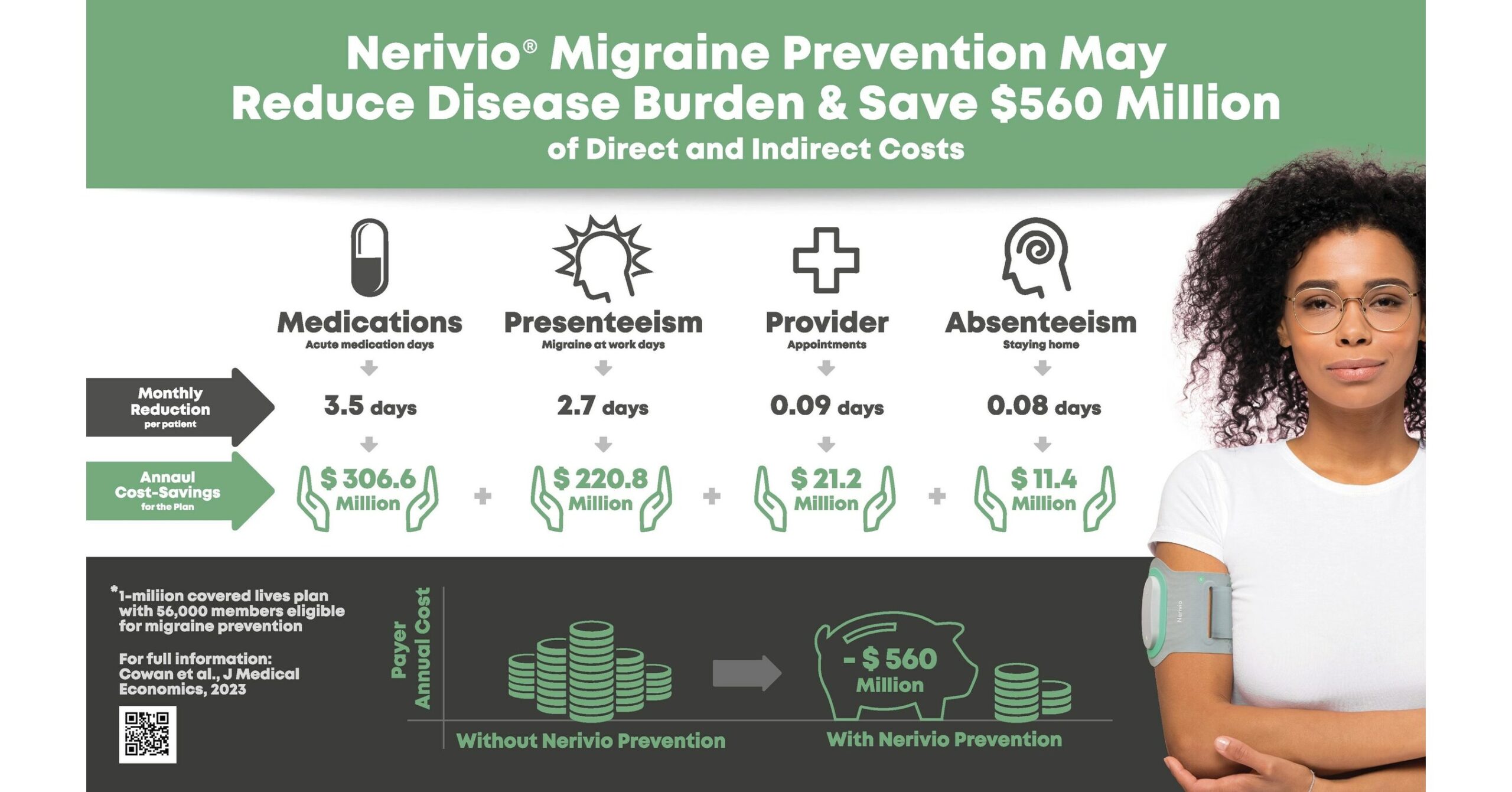
Nerivio, the first and only drug-free, non-disruptive migraine bioband indicated by the FDA for acute and/or preventive treatment of migraine with or without aura in adolescents and adults, was found to reduce disease burden and offer financial incentives for patients, health insurance systems, and employers. The study found that, for a modeled US commercial health plan with one million members, covering Nerivio for all members clinically eligible for preventive treatment of migraine translates to an estimated $10,000 in annual cost savings per member and $560 million in annual savings for the plan.
The randomized, double-blind, placebo-controlled trial aimed to assess the clinical benefits and associated cost savings from use of REN with Nerivio for migraine prevention. The study measured direct healthcare costs, such as reduction in acute medication days, provider visits, and other migraine-related medical care utilization, and indirect costs such as absenteeism and presenteeism (work/school days with moderate to severe functional disability).
The trial consisted of a four-week baseline and eight-week intervention phase, analyzing participants ages 18 to 75 who provided daily reports on these factors. Participants treated with either Nerivio or a placebo device every other day for prevention of migraine.
Nerivio demonstrated statistically significant superiority over placebo in the measurement of key endpoints when used as a preventive treatment, including the following compelling benefits for participants in the second month of treatment:
- Mean reduction of 3.4 acute medication days per month
- Mean reduction of 2.7 presenteeism days per month (the loss of productivity that occurs when people are present at work and school but not able to fully function)
These outcomes translate to significant direct and indirect cost savings for payers and employers. For a one-million-member payer, the largest direct cost-savings constituted a reduction of 2.5 million days of acute migraine medication intake, resulting in $307 million in annual savings for the insurance plan. A decrease in healthcare professional appointments following Nerivio treatment was also higher (albeit not statistically significant) than placebo and contributed an additional average savings of $21 million.
As for indirect cost savings, the largest component came from a decrease in presenteeism, which is especially important for employers. Using Nerivio for migraine prevention was shown in this study to lead to a reduction of about 1.5 working months of presenteeism per person per year, resulting in an average insurance plan savings of roughly $220 million.
“Nerivio’s strong clinical efficacy as an acute and preventive treatment for adolescents and adults is well-documented,” says Robert Cowan, MD, lead author of the study paper, who is a neurologist and Director of Stanford University’s Headache and Facial Pain Research. “There is a pressing need to accelerate value-based care success. This research is a compelling demonstration of how Nerivio is able to lower cost of care, improve clinical outcomes, support preventive measures, and increase patient satisfaction. The real-world implications are important in the context of rising healthcare costs and declining patient satisfaction.”
According to a 2021 consensus statement from the American Headache Society (AHS), only 3 to 13% of individuals with migraine use preventive treatment, even though nearly 40% of those with migraine and nearly all chronic migraine patients would potentially benefit from it.
“We designed Nerivio as a complete, personalized therapy because migraine, being a complex neurological disorder, must be treated holistically, with methods that are preventative first and foremost and seamlessly fit into everyday life,” says Alon Ironi, Theranica CEO and co-founder. “The burden of migraine disease is often debilitating not only physically but also in ways that directly affect people’s quality of life, ability to function at school or work, and need for additional care—all of which drive up costs. The results of this study are a triple win: patients benefit from the reduction in migraine attack frequency and need for acute medications, health insurance plans benefit from the significant direct annual cost savings, and workplaces benefit from the reduction in absenteeism and lost productivity. Health insurance systems and employers should consider the powerful financial incentives that come with this drug-free migraine treatment.”
Nerivio, a migraine bioband, is a digital wearable that wraps around the upper arm and uses sub-painful REN to activate nociceptive nerves fibers in arm to send signals which activate a pain management mechanism in the brain called conditioned pain modulation (CPM), which turns off migraine pain and associated symptoms without medication. Each treatment lasts 45 minutes and is applied every other day for prevention or at the start of a migraine attack for acute treatment.
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable, low-side effect therapies for idiopathic pain conditions. The company’s award-winning flagship wearable, Nerivio®, is the first FDA-cleared, smartphone-controlled, physician-prescribed migraine bioband for acute and preventive treatment of migraine and already serves more than 45,000 people with migraine in the USA, including adolescents and veterans. Theranica is expanding its proprietary technology to develop solutions for additional idiopathic pain conditions. Learn more by visiting our websites, theranica.com and nerivio.com, and following us on LinkedIn, Twitter, Instagram and Facebook.
Theranica Contact
Ronen Jashek
[email protected]
+972-72-390-9750
Media Contact
Morgan O’Donnell
Grey Matter Marketing
[email protected]
SOURCE Theranica