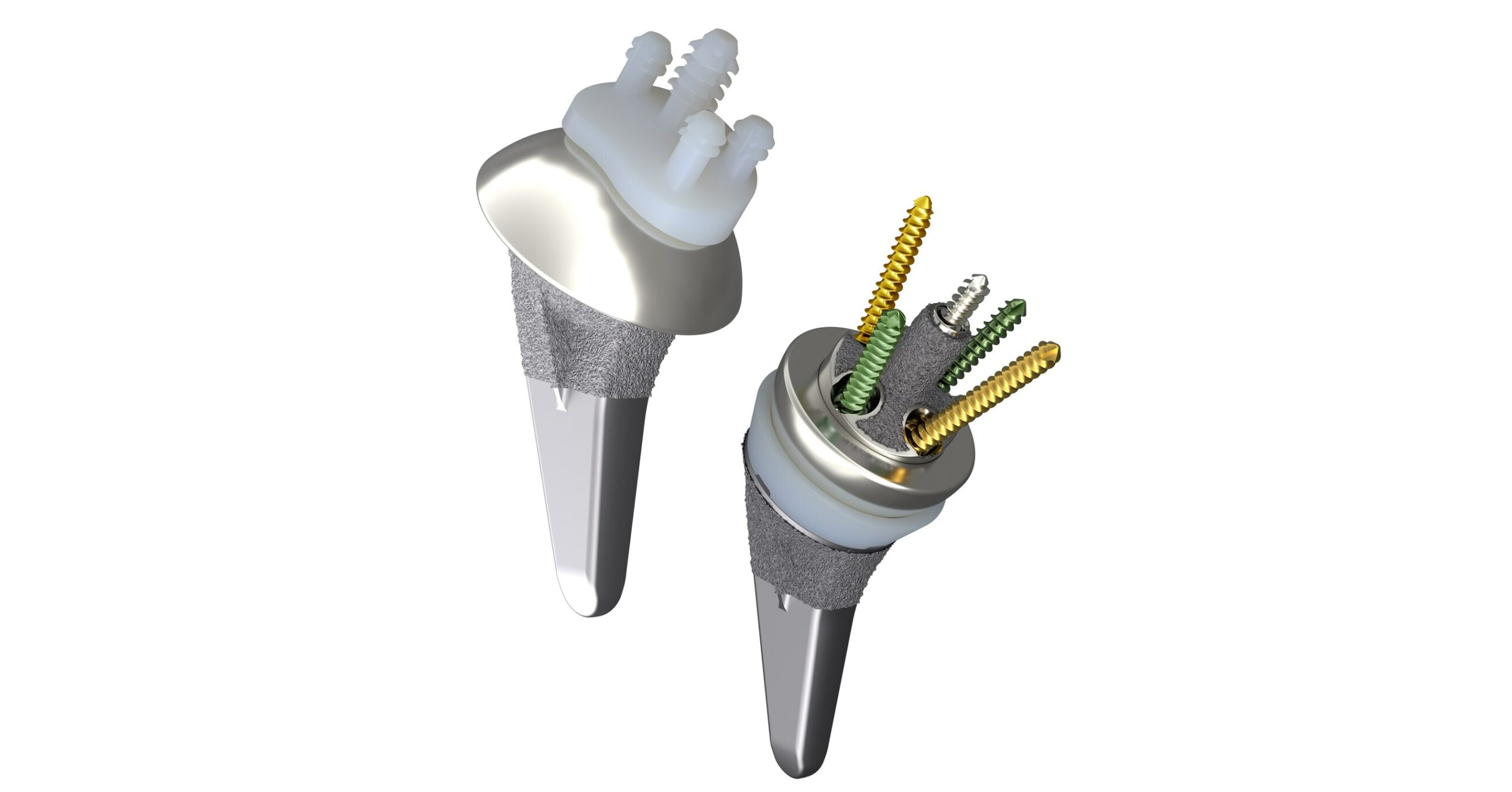
Smith+Nephew announces 510(k) clearance of its AETOS™ Shoulder System for anatomic and reverse shoulder replacement
LONDON, June 12, 2023 /PRNewswire/ — Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its AETOS Shoulder System. Smith+Nephew’s AETOS Shoulder System Designed to restore patients’ range-of-motion1-4 and help minimize arthritic shoulder pain. Features the AETOS Meta Stem – … Read more