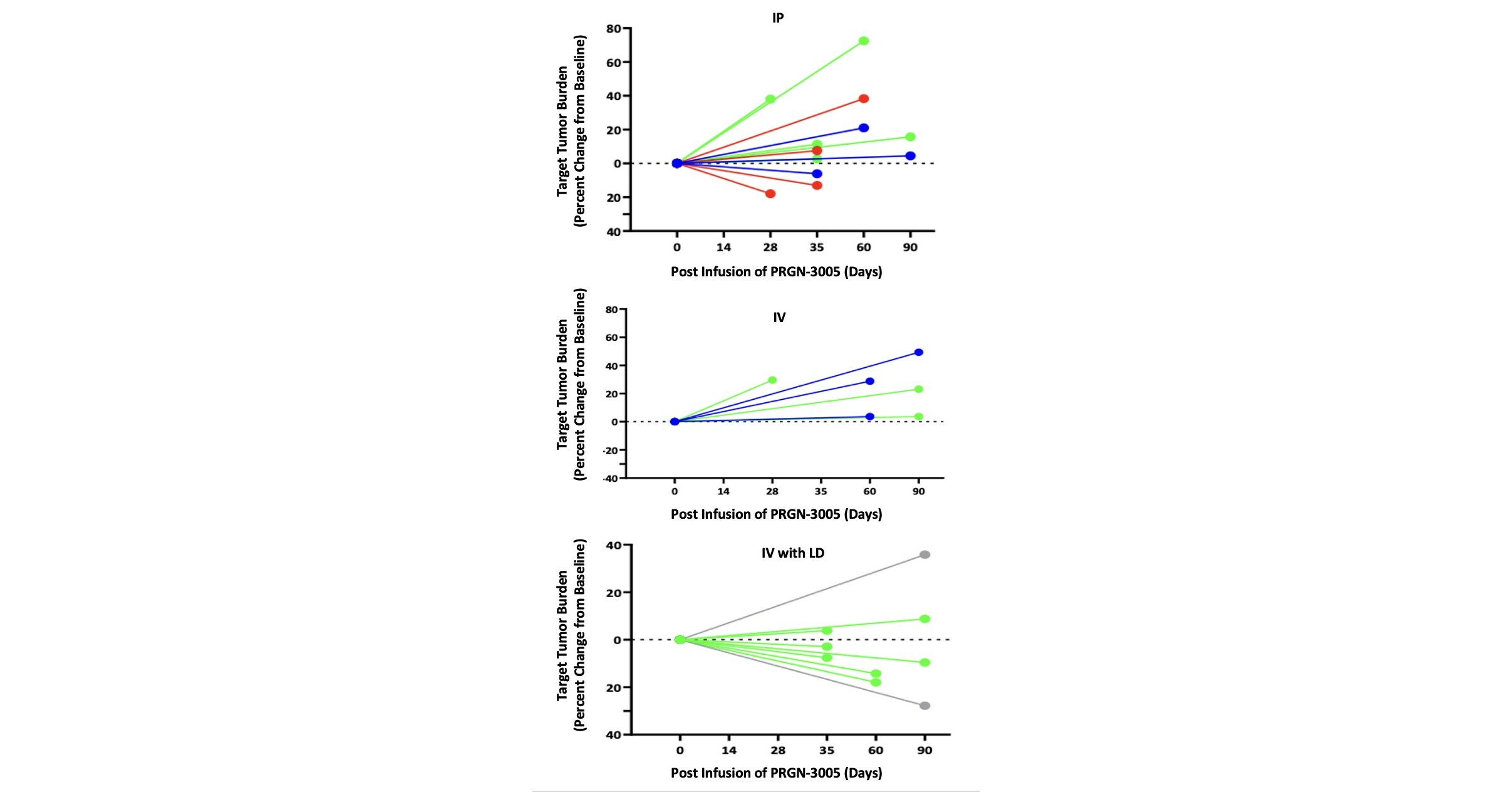
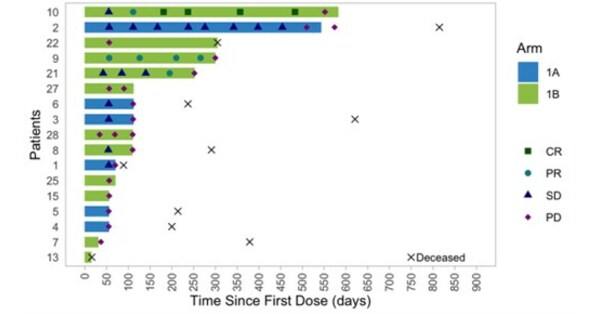
Precigen Receives Breakthrough Therapy Designation for PRGN-2012 AdenoVerse™ Immunotherapy for the Treatment of Recurrent Respiratory Papillomatosis
– First FDA Breakthrough Therapy Designation granted for AdenoVerse immunotherapy platform; designation prioritizes PRGN-2012 as a potential treatment for RRP –– Designation based on positive Phase 1 clinical data that showed 50% of patients were “surgery-free” (Complete Response) after PRGN-2012 treatment with a minimum follow up of 12 months post-treatment – GERMANTOWN, Md. , June 20, … Read more