IBC Launches Full Range of Best-in-Class Molecular Recognition Technology™ (MRT™) Flowsheets for Highly Selective Separations of Key Radionuclides and Preparation of Novel Chelating Agents for Radionuclide Incorporation into Radiopharmaceuticals USA – English India – English
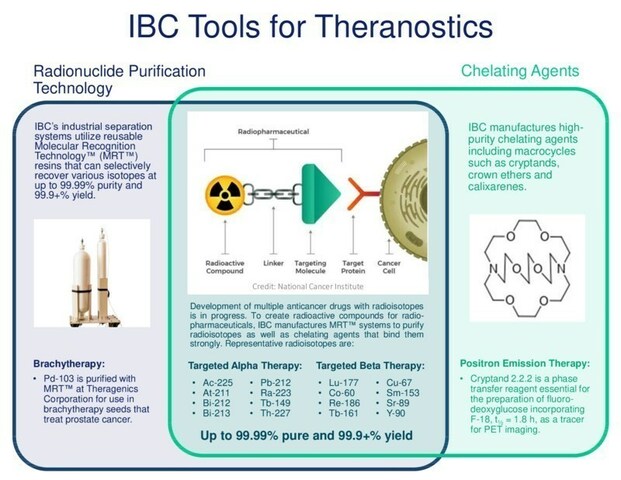
Development of multiple anticancer drugs with radioisotopes is in progress Lu-177, Y-90, Cu-67, Ra-223, Ac-225, Pb-212 and At-211 have licensed products or are in clinical trials IBC’s state-of-the-art MRT™ separation and chelation technologies facilitate production of radiopharmaceuticals based on these and other medical radioisotopes Radioisotopes are separated at up to 99.99% purity and 99.9+% recovery … Read more