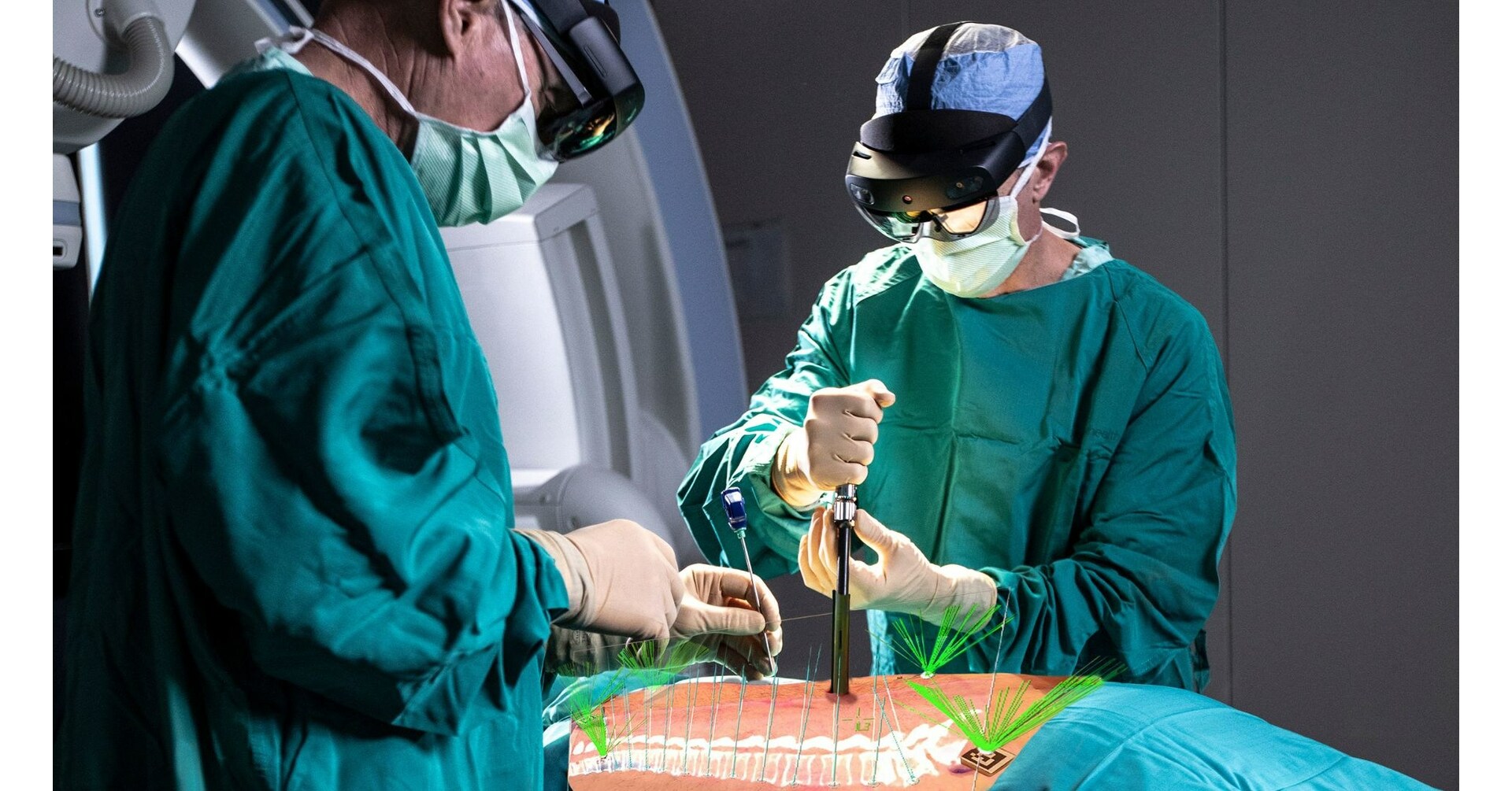
SALT LAKE CITY, May 12, 2023 /PRNewswire/ — Novarad’s VisAR, a surgical navigation system that uses augmented reality, has received clearance from Indonesia’s FDA for intraoperative use in stereotactic spinal surgery. VisAR is accurate for both open and minimally invasive surgery (MISS). This cutting-edge technology enables surgeons to transform a patient’s imaging data into a 3D hologram, which can be projected onto the patient’s body with extreme precision. This allows surgeons to concentrate solely on the surgical objective without requiring them to look away towards a separate monitor, providing accurate surgical guidance.
The VisAR system is a comprehensive solution that includes pre-surgical planning, virtual annotations, segmentation, as well as two-way image connectivity. It also provides integrated 2D and 3D immersive navigation views and ongoing hologram-to-patient registration. VisAR has a sub-2 mm accuracy rate for pedicle screw placement in open and minimally invasive surgical procedures. With a fast a seamless set-up, VisAR enables the surgeon to view the entire OR footprint with voice-controlled commands.
VisAR’s state-of-the-art technology employs CT fiducial markers visible in medical images for automatic registration. This groundbreaking technology is revolutionizing surgical programs worldwide and is becoming a distinguishing factor among healthcare systems.
Novarad has collaborated with Microsoft to utilize pre-built AR headset technology, allowing for lower cost and the ability to leverage expected hardware advancements. With VisAR, physicians wear the wireless Microsoft HoloLens 2 visor and have no need for any other navigational equipment. The use of VisAR technology results in the smallest OR footprint of any system on the market. VisAR is just one of many products in Novarad’s imaging technology solution stack, which delivers interoperability, HIPAA compliance, image management, and comprehensive security features.
“We are thrilled to bring VisAR’s revolutionary technology to Indonesia,” said Dr. Wendell Gibby, Novarad CEO and co-creator of VisAR. “VisAR’s FDA approval in Indonesia is a testament to its innovative design and ability to improve patient outcomes. This technology will enable surgeons in Indonesia to perform complex procedures with greater accuracy and less risk, ultimately benefiting patients.”
VisAR is currently available in the United States and now in Indonesia, with other countries expected to approve the technology in upcoming months. Visit novarad.net/visar for information or to schedule a demonstration.
About Novarad
For over 30 years, Novarad has delivered innovative industry-leading medical technologies that truly transform healthcare for providers and their patients. The enterprise imaging and departmental solutions from Novarad enable thousands of hospitals, imaging centers, and clinics to achieve even greater clinical, operational, and fiscal excellence. Visit Novarad at novarad.net.
SOURCE Novarad Corporation