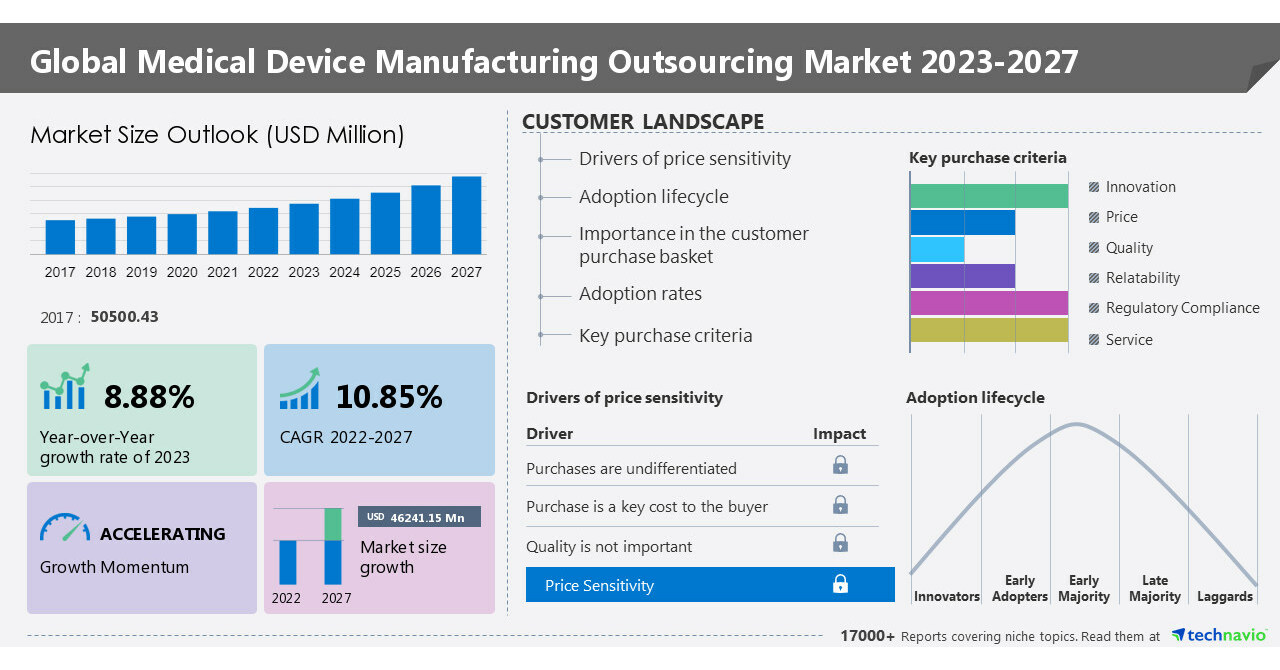
Medical Device Manufacturing Outsourcing Market size to grow by USD 46,241.15 million from 2022 to 2027; Growing focus of OEMs on reducing medical device manufacturing costs
NEW YORK, May 12, 2023 /PRNewswire/ — The medical device manufacturing outsourcing market is set to grow by USD 46241.15 million from 2022 to 2027 progressing at a CAGR of 10.85% during the forecast period. The report offers an up-to-date analysis regarding the current global market scenario, the latest trends and drivers, and the overall market environment. The market is … Read more