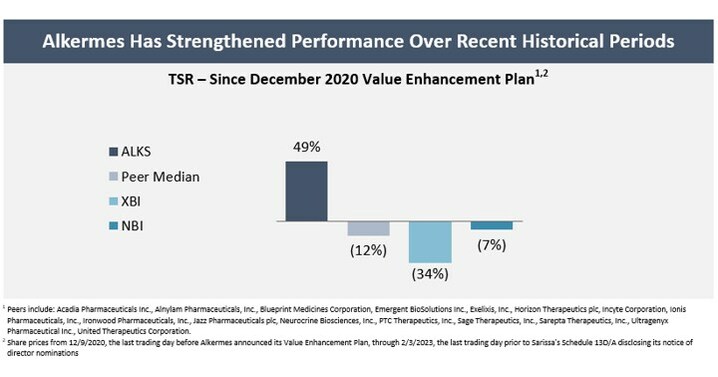
Company Has Outperformed Peers and Relevant Indices Since Unveiling its December 2020 Value Enhancement Plan
70% of Board’s Independent Directors Have Been Appointed Over the Last Four Years, Including Four Directors Designated or Supported by Shareholders
Company Launches www.AlkermesValue.com, Providing Additional Information for Shareholders
Alkermes’ Board Recommends Shareholders Vote “FOR” Alkermes’ Director Nominees on Company’s WHITE Proxy Card
DUBLIN, May 25, 2023 /PRNewswire/ — Alkermes plc (Nasdaq: ALKS) (the Company) today filed its definitive proxy statement with the U.S. Securities and Exchange Commission (SEC) and issued a letter to its shareholders in connection with the Company’s upcoming 2023 Annual General Meeting of Shareholders (the Annual Meeting), which is scheduled to be held on June 29, 2023. The letter details the Company’s successful execution and value creation since the announcement of its Value Enhancement Plan in December 2020. The Value Enhancement Plan is comprised of a number of initiatives, including Board refreshment, evaluation of strategic opportunities and establishment of long-term profitability targets. Key highlights from the letter include that Alkermes:
- Generated a share price increase of 49% since the Company announced its Value Enhancement Plan on December 10, 2020. Since then, the Company’s total shareholder return (TSR) has outperformed its peers1 by 61%, the XBI biotech index by 83% and the NBI biotech index by 56%.2 Alkermes’ TSR has also outperformed its peers and the XBI and NBI on a 1-year and 3-year trailing basis and other relevant timeframes.
- Appointed seven new independent directors to the Company’s board of directors (the Board) over the last four years, while five longer-serving directors have retired. The newly appointed directors, which comprise 70% of the Board’s independent directors, include a director designated by activist hedge fund Sarissa Capital Management LP (Sarissa) in November 2021 (Cato T. Laurencin, M.D., Ph.D.), a director designated by Elliott Advisors (UK) (Elliott) in May 2021 (Emily Peterson Alva), and two directors appointed with the support of Elliott in December 2020 (David A. Daglio and Brian P. McKeon).
- Enhanced the Company’s corporate governance based on shareholder feedback, the evolving needs of the business and trends in governance best practices. These actions included, among others: declassifying the Board and establishing a plurality voting standard for contested director elections; forming the Financial Operating Committee of the Board to oversee achievement of the Company’s profitability targets; refreshing the Board’s Lead Independent Director and the membership and leadership of the Nominating and Corporate Governance Committee of the Board; enhancing the Company’s executive compensation programs; and limiting the number of public company boards on which directors may serve.
- Expanded its commercial portfolio by securing regulatory approvals of two new Alkermes-developed products (LYBALVI® and VUMERITY®) and grew net sales of its proprietary products, and advanced and expanded its pipeline under a disciplined and integrated development approach.
- Initiated the separation of the Company’s oncology business from its neuroscience business and, in connection with this transaction, submitted a confidential draft Form 10 in April 2023.
- Drove strong performance and business momentum through a focus on operational efficiency, disciplined capital allocation, and profitability, including by calibrating Alkermes’ cost structure to appropriately support the Company’s strategic priorities and growth opportunities.
Despite the Company’s positive momentum and strong performance over this timeframe, Sarissa notified Alkermes in February 2023 of its intent to nominate three director candidates for election at the Annual Meeting.
The Board recommends that shareholders vote “FOR” all seven of the Board’s director nominees – Emily Peterson Alva, Cato T. Laurencin, M.D., Ph.D., Brian P. McKeon, Christopher I. Wright, M.D., Ph.D., Shane M. Cooke, Richard B. Gaynor, M.D. and Richard F. Pops – using the Company’s WHITE proxy card. The Company’s proxy statement and other important information and resources related to the Annual Meeting can be found at www.AlkermesValue.com or investor.alkermes.com/investor-relations.
The full text of the letter being sent to shareholders follows:
Dear Alkermes Shareholder,
Your vote at the Alkermes plc (the “Company” or “Alkermes”) Annual General Meeting of Shareholders (the “Annual Meeting”) on June 29, 2023 is very important.
Alkermes is a fully integrated, global biopharmaceutical company focused on developing innovative new medicines to address unmet patient needs and on driving long-term, sustainable value for its shareholders. In 2019, driven by the Company’s share performance, operational considerations, and shareholder feedback, the Company’s board of directors (the “Board”) and management recognized the need to realign the Company’s priorities, refine its strategic and operational focus, and effect certain governance changes. The Board initiated significant Board refreshment efforts and, in December 2020, announced its Value Enhancement Plan, which is comprised of a number of initiatives, including continued Board refreshment, evaluation of strategic opportunities and establishment of long-term profitability targets.
Alkermes’ strong performance since the announcement of the December 2020 Value Enhancement Plan is a direct result of the Company’s execution against its three strategic priorities, as overseen by its recently refreshed Board:
- Growing its portfolio of proprietary commercial products.
- Advancing its development pipeline.
- Driving profitability for the benefit of its shareholders.
For each of these strategic priorities, Alkermes has established clear objectives designed to drive shareholder value, including, for 2023:
- Drive the launch and continued uptake of LYBALVI® through execution of the Company’s commercial strategy.
- Advance ALKS 2680, the Company’s orexin 2 receptor agonist, including by establishing its initial safety and tolerability profile and generating clinical proof-of-concept data.
- Separate the Company’s oncology business from its neuroscience business, which the Board believes will unlock shareholder value by enhancing the performance of both businesses and accelerating profitability for the neuroscience business.
Underpinning these priorities and objectives and the operation of the business is the Company’s foundation of strong corporate governance, dedication to patients and employees, and its commitment to operating in an ethical and responsible manner.
ALKERMES HAS HAD STRONG RECENT PERFORMANCE AND MOMENTUM
The Company has been focused on evolving its business and establishing a strong foundation to drive shareholder value. These efforts began prior to activist hedge fund Sarissa Capital Management LP (“Sarissa”) becoming a shareholder of Alkermes. Since the Company announced its Value Enhancement Plan in December 2020, Alkermes’ share price has increased by 49%, and the Company has outperformed its peers1 by 61%, the XBI biotech index by 83% and the NBI biotech index by 56%.2 Alkermes’ total shareholder return (“TSR”) has also outperformed its peers and the XBI and NBI over various other timeframes, including on a 1-year and 3-year trailing basis.
Since the announcement of the December 2020 Value Enhancement Plan, the Company’s enterprise value to a next twelve-month revenue multiple has increased by approximately 51% from 2.5x to 3.8x, reflecting investors’ recognition of the Company’s performance and future prospects.
Despite the Company’s positive momentum and strong execution of its business strategy over this timeframe, Sarissa notified Alkermes in February 2023 of its intent to nominate three director candidates for election at the Annual Meeting. This is the third year in a row Sarissa has submitted nominations to the Company, following a settlement in 2021 that resulted in the appointment to the Board of a Sarissa-designated director (who continues to serve on the Board) and Sarissa’s abandonment of its previous proxy campaign in 2022.
Earlier this year, the Board conducted its annual evaluation of the desired mix of skills, experiences and diversity of perspectives to support the Company’s strategic priorities, and the four independent directors on the Board’s Nominating and Corporate Governance Committee interviewed and carefully considered all of Sarissa’s candidates. Following the committee’s evaluation, the Board determined that the key attributes and experience of the three Sarissa nominees were neither additive to the Board at this time nor consistent with the skillset previously identified by the Board as important in a new director. The Board therefore determined that appointing Sarissa’s nominees as directors would not be in the best interests of the Company and its shareholders. Notwithstanding the foregoing decision, the Alkermes Board has made numerous attempts to reach a resolution with Sarissa and a number of the Company’s independent directors have continued to discuss with Sarissa ways to prevent a proxy contest.
ALKERMES HAS A REFRESHED, HIGHLY ENGAGED, DIVERSE AND INDEPENDENT BOARD WITH STRONG GOVERNANCE
Alkermes has active, skilled and highly experienced Board members who are deeply engaged in oversight of the Company and its strategy. Starting in 2019, the Board initiated significant Board refreshment efforts and implemented new initiatives to enhance its corporate governance, its compensation programs and its business. Since that time, Alkermes has appointed seven new independent directors to the Board and five longer-serving directors have retired. None of the new, independent directors appointed had any previous connections to the Company or its leadership team. These new directors, who comprise 70% of the Board’s independent directors, include a director designated by Sarissa in November 2021 (Cato T. Laurencin, M.D., Ph.D.), a director designated by Elliott in May 2021 (Emily Peterson Alva), and two directors appointed with the support of Elliott in December 2020 (David A. Daglio and Brian P. McKeon). In 2022, in connection with its Board refreshment efforts, Alkermes appointed Nancy J. Wysenski as its new Lead Independent Director.
The Board refreshment efforts added diverse backgrounds, skills, experiences, and perspectives to the Board that are relevant to the Company’s industry and operations and aligned with its business strategy. The Board is comprised of individuals with extensive experience in a variety of areas that are critical to the Company’s business strategy, including life sciences industry experience; commercial marketing and sales experience; finance and accounting experience; management and governance experience; buyside investor perspective and experience; and corporate strategy and complex transactional experience.
Alkermes maintains strong corporate governance and Board oversight practices that promote the long-term interests of its shareholders. The Company and the Board regularly engage with shareholders in respect of governance and other matters and the Board continually reviews and, as it deems appropriate, refines its governance policies and practices. As a result of shareholder feedback, the evolving needs of the business and trends in governance best practices, the Board has, in recent years, taken the following actions, among others, to enhance Alkermes’ corporate governance, including:
- Declassified the Board: In July 2020, the Company announced its plans to declassify the Board and, at the Company’s 2021 annual meeting, the Company’s shareholders approved the declassification of the Board over a three-year period. This process will be completed next year.
- Adopted Plurality Voting for Contested Elections: In May 2022, the Board convened an Extraordinary General Meeting of Shareholders at which, at the Board’s recommendation, the Company’s shareholders approved the establishment of a plurality voting standard for contested director elections.
- Formed the Financial Operating Committee of the Board: In December 2020, the Board formed the Financial Operating Committee of the Board, on which all three Elliott-supported directors serve, to oversee achievement of the Company’s profitability targets and evaluate a broad range of potential strategic options related to the Company’s non-core assets, including potential monetization and divestiture opportunities.
- Refreshed the Nominating and Corporate Governance Committee: During 2022, the Board added three new independent committee members and appointed a new committee chair. All four members of the committee are diverse in terms of gender and/or race/ethnicity. Two members of the committee, including Sarissa’s director designee, were appointed to the Board in collaboration with shareholders.
- Enhancements to Executive Compensation Programs: Since 2019, the Board has engaged in a concerted multi-year effort to solicit and respond to shareholder feedback related to the Company’s executive compensation programs. Shareholder feedback received through these engagement efforts led to the implementation of meaningful changes to Alkermes’ executive compensation programs and other governance changes.
- Limited the Number of Public Company Boards on Which Directors May Serve: In May 2022, the Board revised its “overboarding” policy to further limit the number of public company boards on which the Company’s directors may serve, including that the Company’s CEO or other employee directors may serve on a maximum of one outside public company board and the Company’s non-employee directors may serve on a maximum of three other public company boards (in addition to the Alkermes Board).
ALKERMES EXPANDED ITS PORTFOLIO OF PRODUCTS AND ADVANCED ITS PIPELINE PROGRAMS
Alkermes has been consistently executing on numerous initiatives to drive shareholder value. The Company has successfully expanded its commercial portfolio by securing regulatory approvals of two new Alkermes-developed products, grown its revenues from proprietary products, and advanced and expanded its pipeline under a new, disciplined development approach. These achievements include:
Two New Product Approvals
- In 2021, secured U.S. Food and Drug Administration (“FDA”) approval and launched LYBALVI, an oral medicine for the treatment of schizophrenia and bipolar I disorder.
- In 2019, secured FDA approval of VUMERITY® for the treatment of relapsing forms of multiple sclerosis, and supported Biogen’s commercial launch of this product.
Growth of Proprietary Commercial Product Portfolio
- Grew proprietary commercial product net sales from $524 Million in 2019 to $777 million in 2022, representing growth of 48%.
- Generated strong initial uptake of LYBALVI that exceeded analyst expectations in the first year of launch; through the first quarter of 2023, more than 9,300 healthcare providers had written a prescription for LYBALVI and more than 115,000 prescriptions for LYBALVI had been dispensed.
- Re-established VIVITROL® growth in the addiction treatment system following COVID-19-related disruptions, and redefined VIVITROL’s commercial strategy in the alcohol dependence indication.
- Drove growth of ARISTADA®, including, most recently, 10% year-over-year growth in the first quarter of 2023.
Advancement of Neuroscience and Oncology Development Pipeline
- Initiated phase 1 first-in-human study for ALKS 2680, Alkermes’ orexin 2 receptor agonist, enrolling initial cohorts and dosing subjects well ahead of anticipated timelines, with initial clinical proof-of-concept data expected before year-end 2023.
- Advanced nemvaleukin alfa (“nemvaleukin”) into potential registrational trials in two tumor types and secured both FDA Fast Track designation for nemvaleukin in mucosal melanoma and in combination with pembrolizumab in platinum-resistant ovarian cancer, and FDA Orphan Drug designation for nemvaleukin in mucosal melanoma.
Active Management of Royalty and Manufacturing Business
- Secured multiple successful interim awards in the Company’s binding arbitration proceedings against Janssen Pharmaceutica N.V. (“Janssen”), a subsidiary of Johnson & Johnson, related to its partial termination of two license agreements with the Company and Janssen’s decision to cease paying royalties on certain products in the U.S. Pursuant to the interim awards, the arbitration panel found that, notwithstanding its termination of the license agreements, Janssen must continue to pay the Company royalties on the applicable products in the U.S. and that the Company is entitled to such royalties for 15 years from first commercial sale of each such product. Sarissa previously cited the Janssen dispute as a reason for deciding to submit a nomination notice in 2022. Alkermes expects to receive a final award in due course and plans to update its 2023 financial expectations accordingly.
THE ALKERMES BOARD AND MANAGEMENT TEAM HAVE BEEN DRIVING CHANGE FOCUSED ON OPERATIONAL EFFICIENCY, DISCIPLINED CAPITAL ALLOCATION, AND PROFITABILITY
The Board and Alkermes’ management team have been keenly focused on the following objectives to best position Alkermes for long-term growth and value creation:
- Execute on the Company’s December 2020 Value Enhancement Plan.
- Drive operational efficiency and calibrate Alkermes’ cost structure to appropriately support the Company’s strategic priorities and growth opportunities.
- Work toward the planned separation of the oncology business.
Alkermes has taken substantial actions to optimize its cost structure over the past four years in response to the evolving needs of the Company and shareholder feedback, and in support of its commitment to driving shareholder value creation.
Value Enhancement Plan. The December 2020 Value Enhancement Plan is comprised of a number of initiatives, including Board refreshment, evaluation of strategic opportunities and establishment of long-term profitability targets. This Plan was informed by direct engagement with, and feedback from, many of Alkermes’ largest institutional shareholders throughout 2019 and 2020. Announcement of the Plan followed constructive dialogue and entry into a cooperation agreement with Elliott.
Cost Structure Optimization. In 2019, prior to its interactions with Sarissa or Elliott, Alkermes implemented a restructuring, which included headcount reductions and other cost-saving measures. In addition, in 2020, the Company adopted a revised research and development (“R&D”) capital allocation strategy focused on prioritizing R&D programs that it believes offer the highest potential return on investment. That disciplined strategy resulted in the discontinuation of several early-stage development programs based on data-driven decision making against pre-defined success criteria and R&D stage-gates. In 2020, Alkermes implemented a reorganization of the Company’s commercial organization in preparation for the launch of LYBALVI, consolidating several functional areas to drive operating leverage and allocating resources to effectively drive growth.
Separation of the Oncology Business. In November 2022, following a robust evaluation process, Alkermes announced the planned separation of its oncology business from its neuroscience business. The Company submitted a confidential draft Form 10 for this transaction in April 2023 and expects the separation to be completed in the second half of 2023. The Board unanimously agreed that the unique needs of each business would be best served by simplified resource and capital allocation decision making, tailored operating structures, and distinct leadership teams, each with a clearly defined strategic focus. Following the planned separation of the oncology business, Alkermes will be a pure-play, commercial-stage neuroscience company, retaining its focus on significant unmet needs within neuroscience and on driving growth of its proprietary commercial products: LYBALVI, ARISTADA/ARISTADA INITIO®, and VIVITROL. The separated oncology business, recently named Mural Oncology, will be a pure-play oncology company, focused on the discovery and development of cancer therapies, including the continued development of nemvaleukin and the Company’s portfolio of novel, preclinical engineered cytokines, comprised of tumor-targeted split interleukin-12 (IL-12) and interleukin-18 (IL-18).
Accelerated Profitability Targets*. In February 2023, the Company accelerated its long-term profitability targets to reflect the anticipated financial benefit of the planned separation of the oncology business in the second half of 2023, and announced its commitment to achieving the following:
- FY 2024 non-GAAP net income equal to 25% of the Company’s total revenues and EBITDA3 margin of 20% of total revenues
- FY 2025 non-GAAP net income equal to 30% of the Company’s total revenues and EBITDA margin of 25% of total revenues
The Company did not provide reconciliations of, or comparable GAAP measures for, the above non-GAAP profitability targets, as they are not determinable without unreasonable efforts.
ALKERMES’ PROGRESS, BUSINESS MOMENTUM, AND POTENTIAL HAS BEEN RECOGNIZED BY SELL-SIDE ANALYSTS
Recent commentary from sell-side analysts includes:
“ALKS clearly has the flexibility to not only effectuate the spin-out of a funded oncology business but also execute on the addition of assets that can bolster the neuropsychiatry pipeline. We believe that ALKS is well-positioned for meaningful further value creation.”
– David Amsellem, Piper Sandler (April 26, 2023)
“Regarding the arbitration with J&J, we are not surprised that Alkermes won this case. We have never understood why J&J believed that it didn’t owe Alkermes the appropriate and usual CM/royalties and that it could try to get out of this agreement. . . Regarding the decision to hive off the oncology business, we remain very supportive that this is a good move.”
– Marc Goodman, SVB Securities (April 26, 2023)
“We remain bullish on ALKS. We continue to believe the planned separation would unlock value for the oncology business and the standalone neuroscience company, transforming it into a more attractive entity given the potential blockbuster opportunity from LYBALVI and ALKS 2680 (orexin 2 agonist) and the leaner operating cost structure.”
– Uy Ear, Mizuho (April 26, 2023)
“Outperformance has been delivered, in our view, on an attractive combination of commercial delivery (solid 4Q22 commercial results and FY2023 guidance), the declaration of a more focused strategy (with the planned separation of the oncology and neuroscience businesses), and the emergence of a pipeline asset, ALKS 2680.”
– Chris Shibutani, MD, Goldman Sachs (April 18, 2023)
“ALKS put up one of its better Q’s in yrs. The ’23 guide was solid–LYBALVI guide was ~5% higher vs cons. & implies inflection in ’23 likely on the back of DTC efforts…. This remains the best idea in our coverage given base business execution + blue sky upside w/OX2.”
– Akash Tewari, Jefferies (February 16, 2023)
“We agree with ALKS’s decision to focus the business on neuroscience…we see the company on the cusp of a significant transition with announced plans to separate the oncology effort into a separate business allowing more direct focus on achieving improved profitability for the neuroscience business.”
– Jessica Fye, JP Morgan (February 14, 2023)
PLEASE VOTE USING THE COMPANY’S WHITE PROXY CARD TODAY
The Board has nominated seven director nominees for re-election to the Board at the Annual Meeting: Emily Peterson Alva, Shane M. Cooke, Richard B. Gaynor, M.D., Cato T. Laurencin, M.D., Ph.D., Brian P. McKeon, Richard F. Pops and Christopher I. Wright, M.D., Ph.D. The Board recommends that shareholders vote ‘FOR’ all seven of the Board’s director nominees using the WHITE proxy card. The Company’s proxy statement and other important information and resources related to the Annual Meeting can be found at www.AlkermesValue.com.
There are three easy ways to vote:
|
BY INTERNET |
BY TELEPHONE |
BY MAIL |
|
Visit the website shown on your |
Dial the toll-free number shown on |
Mark, date, sign and return the |
If you have any questions about how to vote your shares, or need assistance in voting, please contact the firm assisting Alkermes with the solicitation of proxies for the Annual Meeting:
Innisfree M&A Incorporated
Toll-Free at (877) 750-8334 (toll-free for those calling from the U.S. and Canada) or
+1 (412) 232-3651 (for those calling from outside the U.S. and Canada)
To learn more about Alkermes’ Board nominees, business strategy, and strong recent performance, please visit www.AlkermesValue.com.
Alkermes looks forward to communicating with you further as the Annual Meeting approaches, and as always, appreciates your continued support.
Sincerely,
The Alkermes Board of Directors
About Alkermes plc
Alkermes plc is a fully-integrated, global biopharmaceutical company developing innovative medicines in the fields of neuroscience and oncology. The company has a portfolio of proprietary commercial products focused on alcohol dependence, opioid dependence, schizophrenia and bipolar I disorder, and a pipeline of product candidates in development for cancer and neurological disorders. Headquartered in Dublin, Ireland, Alkermes has a research and development center in Waltham, Massachusetts; a research and manufacturing facility in Athlone, Ireland; and a manufacturing facility in Wilmington, Ohio. For more information, please visit Alkermes’ website at www.alkermes.com.
Forward-Looking Statements
Certain statements set forth in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning the Company’s expectations concerning its future financial and operating performance, business plans or prospects, including its ability to execute on its strategy and create and deliver growth and shareholder value and its ability to achieve long-term profitability and its profitability targets; the Company’s engagement with Sarissa; expectations with respect to the final award in the Company’s arbitration proceedings with Janssen and the Company’s plans to update its 2023 financial expectations; expectations regarding the timing, structure, anticipated benefits and other impacts of the planned separation of the Company’s oncology business; timelines, plans and expectations for development activities relating to ALKS 2680; and the therapeutic and commercial potential of the Company’s products. The Company cautions that forward-looking statements are inherently uncertain. The forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks and uncertainties, including that the Company may not ultimately separate its oncology business during 2023 or at all; unanticipated developments, costs or difficulties that may delay or otherwise negatively affect a potential separation of the Company’s neuroscience and oncology businesses; the Company may not be able to achieve long-term profitability or its profitability targets in a timely manner or at all; the terms of the final award to be issued in the Company’s arbitration proceedings with Janssen may differ from the terms of the interim awards issued in such arbitration proceedings and may be challenged by Janssen; clinical development activities may not be completed on time or at all; the results of the Company’s development activities may not be positive, or predictive of final results from such activities, results of future development activities or real-world results; the FDA or regulatory authorities outside the U.S. may not agree with the Company’s regulatory approval strategies or components of the Company’s marketing applications; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the Company’s products; the Company and its licensees may not be able to continue to successfully commercialize their products or support revenue growth from such products; the Company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks and uncertainties described under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended Dec. 31, 2022 and in subsequent filings made by the Company with the SEC, which are available on the SEC’s website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the Company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release.
Important Additional Information and Where to Find It
The Company has filed its definitive proxy statement, accompanying WHITE proxy card and other relevant documents with the SEC in connection with the solicitation of proxies for the Annual Meeting. BEFORE MAKING ANY VOTING DECISION, SHAREHOLDERS OF THE COMPANY ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE COMPANY’S DEFINITIVE PROXY STATEMENT AND ANY AMENDMENTS AND SUPPLEMENTS THERETO, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Investors and shareholders will be able to obtain a copy of the definitive proxy statement and other documents filed by the Company with the SEC free of charge from the SEC’s website at www.sec.gov. In addition, copies will be available at no charge by visiting the “Investors” section of the Company’s website at www.alkermes.com, as soon as reasonably practicable after such materials are filed with, or furnished to, the SEC.
Certain Information Regarding Participants in the Solicitation
The Company, its directors and certain of its executive officers are participants in the solicitation of proxies from shareholders in respect of the Annual Meeting. Information regarding the names of such participants and their respective interests in the Company, by security holdings or otherwise, is set forth in the Company’s definitive proxy statement for the Annual Meeting, which can be obtained free of charge from the sources indicated above.
Non-GAAP Financial Measures
This press release includes information about certain financial measures that are not prepared in accordance with GAAP, including non-GAAP net income margin (non-GAAP net income/total revenue) and EBITDA margin (EBITDA/total revenue). These non-GAAP measures are not based on any standardized methodology prescribed by GAAP and are not necessarily comparable to similar measures presented by other companies. Non-GAAP net income adjusts for certain one-time and non-cash charges by excluding from GAAP results: share-based compensation expense; amortization; depreciation; non-cash net interest expense; change in the fair value of contingent consideration; certain other one-time or non-cash items; and the income tax effect of these reconciling items.
The Company’s management and Board utilize these non-GAAP financial measures to evaluate the Company’s performance. The Company provides these non-GAAP financial measures of the Company’s performance to investors because management believes that these non-GAAP financial measures, when viewed with the Company’s results under GAAP and the accompanying reconciliations, are useful in identifying underlying trends in ongoing operations. However, non-GAAP net income margin and EBITDA margin are not measures of financial performance under GAAP and, accordingly, should not be considered as alternatives to GAAP measures as indicators of operating performance. Further, non-GAAP net income margin and EBITDA margin should not be considered measures of the Company’s liquidity.
*The Company has not provided full financial expectations for time periods after the year ending Dec. 31, 2023 and therefore is not providing reconciliations of, or comparable GAAP measures for, non-GAAP net income margins or EBITDA margins, for time periods after the year ending Dec. 31, 2023. Reconciliations of such forward-looking non-GAAP profitability measures to comparable GAAP measures are not determinable without unreasonable efforts due to the inherent difficulty in forecasting and quantifying certain future financial amounts necessary for such reconciliations, which amounts could have a significant impact on the Company’s future financial results, including such non-GAAP profitability measures and the comparable GAAP financial measures.
Contacts:
For Investors: Sandy Coombs, +1 781 609 6377
For Media: Katie Joyce, +1 781 249 8927
Or
FGS Global
Chris Kittredge / Zachary Tramonti
[email protected]
|
1 Peers include: Acadia Pharmaceuticals Inc., Alnylam Pharmaceuticals, Inc., Blueprint Medicines Corporation, Emergent BioSolutions Inc., Exelixis, Inc., Horizon Therapeutics plc, Incyte Corporation, Ionis Pharmaceuticals, Inc., Ironwood Pharmaceuticals, Inc., Jazz Pharmaceuticals plc, Neurocrine Biosciences, Inc., PTC Therapeutics, Inc., Sage Therapeutics, Inc., Sarepta Therapeutics, Inc., Ultragenyx Pharmaceutical Inc., United Therapeutics Corporation. |
|
2 Share prices from 12/9/2020, the last trading day before Alkermes announced its Value Enhancement Plan, through 2/3/2023, the last trading day prior to Sarissa’s Schedule 13D/A disclosing its notice of director nominations |
|
3 Calculated as earnings before interest, taxation, depreciation, amortization and one-time items, includes share-based compensation expenses. |
SOURCE Alkermes plc